Copper ii sulfate also known as copper sulphate are the inorganic compounds with the chemical formula cu so 4 h 2 o x where x can range from 0 to 5 the pentahydrate x 5 is the most common form.
The colour of copper sulphate is dash.
Water also comes out of the copper sulfate and is able to function as any other type of water would typically function.
Stir in a small amount of ammonia.
The proportions are not critical but you want a high enough concentration of copper sulfate to get a blue color.
Lalpuranlal939 lalpuranlal939 1 hour ago science.
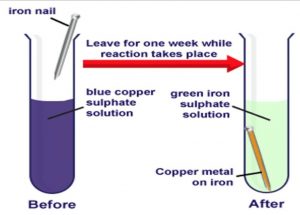
Find an answer to your question why does the colour of copper sulphate solution change when an iron nail is dipped in it 1.
Access detailed answers to various other science and maths questions at byju s.
The pentahydrate cuso 4 5h 2 o the most commonly encountered salt is bright blue.
Older names for this compound include blue vitriol bluestone vitriol of copper and roman vitriol.
For every copper ion cu 2 discharged at the cathode as neutral copper atom cu a copper ion cu 2 is released or added to the solution at the anode and hence the total number of cu 2 ions remains the same.
When heated the salt loses its water of crystallization and turns white.
The flame test is a relatively easy experiment to set up and thus is often demonstrated or carried out in science classes in schools.
Meenakshigupta63 meenakshigupta63 2 days ago chemistry secondary school 50 pts.
Dissolve a spoonful of copper sulfate in a cup of hot water.
The crystals of hydrated copper sulphate salt are blue in colour.
The blue solid will settle out of solution if you allow it to sit.
See the swirls of milky pale blue.
Perform the color change demo.
Copper sulfate is generally a blue color.
It is sometimes called blue copper for this reason.
When the copper sulfate is heated up it quickly turns to a pure white color that has no hint of blue in it.